Introduction:
We previously showed that HIV associated EBV negative (-) diffuse large B-cell lymphomas (DLBCL) of germinal center origins (GCB) have enhanced genomic stability and increased expression of DNA repair genes compared to their HIV negative counterparts (Maguire et al 2019). Given that immune deficiency can be induced virally or therapeutically, including the administration of immunosuppressive drugs post solid organ or hematopoietic stem cell transplant, we questioned whether these observations are specific to HIV or if they represent a more global immune impaired signature. Here we assessed the genomic stability of monomorphic DLBCL post-transplant lymphoproliferative disorders (PTLD) and the potential role of DNA repair.
Methods:
A total of 49 monomorphic DLBCL PTLDs (26 EBV(-), 23 EBV positive (+)) and 170 immune competent (IC) DLBCL (EBV status unknown) were studied. All were pathologically reviewed and samples with <60% tumor content were macro-dissected before RNA/DNA extraction. Cell of origin (COO) profiles were assessed using the DLBCL90 molecular assay which can distinguish primary mediastinal B-cell lymphoma (PMBL) from GCB, Activated B-cell (ABC) and unclassified (UNC) DLBCL (Ennishi et al 2019). NanoString gene expression profiling (GEP) was performed using the PanCancer Pathways panel and analyzed using NanoString nSolver Advanced Analysis. Copy number (CN) analysis was performed on a subset of samples (45 PTLD, 41 DLBCL) using the OncoScan CN assay and analyzed using Chromosome Analysis Suite (ChAS) v4.1. All p-values listed were Benjamini-Hochberg adjusted and Mann-Whitney tested for the GEP and CN data respectively.
Results:
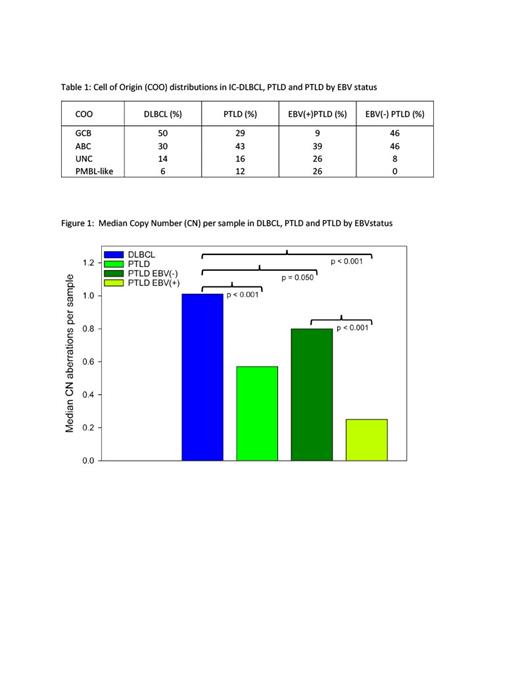
In contrast to IC-DLBCL which are GCB enriched (50%), COO profiling revealed PTLD to be ABC enriched (43%). CN analyses revealed that the median CN/sample for PTLD (0.57, p<0.001), EBV(+)PTLD (0.25, p<0.001) and EBV(-)PTLD (0.80, p = 0.050) tumors were significantly less aberrant than IC-DLBCL (1.01). The data also showed that EBV(+)PTLD are less aberrant than EBV(-)PTLD tumors (0.25 vs 0.80, p<0.001).
GEP pathway analysis identified DNA repair as downregulated in PTLD and EBV(+)PTLD but upregulated in EBV(-)PTLD compared IC-DLBCL. Nine DNA repair genes ( RAD51, CCNA2a, CHEK1b, POLR2Da, PCNAa, WHSC1a, GTF2H3a, ATM c, EP300 c) were differentially expressed (DE) in EBV(-)PTLD compared to IC-DLBCL (7 increased, 2 decreased, p ≤ 0.0425): where the most overexpressed gene RAD51, a critical mediator of Homologous Recombination (HR) and the Fanconi Anemia (FA) DNA repair pathways, was not DE in PTLD or EBV(+)PTLD compared to IC-DLBCL. Five a genes were increased in PTLD (p ≤ 0.0204) but not EBV(+)PTLD, 1 b was decreased in EBV(+)PTLD (p = 0.01) but not PTLD and 2 c were reduced in PTLD regardless of EBV status compared to IC-DLBCL (p ≤ 0.0024).
Twenty-8 genes were DE in EBV(+)PTLD compared to IC-DLBCL. Of these, 23 were downregulated (p ≤ 0.0418) and included HR, FA and mismatch repair genes ( CHEK1, CHEK2, BRAC1/FANCS, FANCA, FANCB, FANCE, MSH2, MSH6). All but 2/23 were similarly expressed in PTLD compared to IC-DLBCL of which 11 were significant (p ≤ 0.0356). The five upregulated genes ( ZBTB32, DDB2, NBN, POLB, RBX1, p ≤ 0.0092) were also upregulated in PTLD (p ≤ 0.0042) but not EBV(-)PTLD compared to IC-DLBCL.
Two additional genes ( SKP1, FBXW7) were also identified as increased in EBV(+)PTLD and PTLD (p ≤ 0.0204) compared to IC-DLBCL. In contrast, SKP1 was increased (p = 2.7E-4) and FBXW7 was decreased (p = 0.3610) in EBV(-)PTLD compared to IC-DLBCL. Together the protein products of RBX1, SKP1 and FBXW7 comprise the functional cores of a complete UPS complex, the SCF FBXW7 E3 ubiquitin ligase which have been reported to regulate the DNA damage response and promote genomic stability.
Conclusions:
Like HIV associated DLBCL, these results show that PTLD is more genomically stable than IC-DLBCL, and that genomic stability is further enhanced in EBV(+)PTLD which have increased expression of UPS related genes. In contrast, EBV(-)PTLD have increased expression of DNA damage repair genes, including RAD51. In summary, monomorphic DLBCL PTLD tumors have enhanced genomic stability that appears to be mediated by two distinct mechanisms stratified by EBV status. Further work is required to assess if this stratification of mechanisms is unique to PTLD or reflects a more global immune impaired EBV related signature.
Disclosures
Rimsza:NanoString: Other: Licensed intellectual property; Roche: Other: Consulting.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal